EndoBarrier Study Shows Improvement in Cardiovascular Risk
BOSTON and SYDNEY — 15 January 2020 — GI Dynamics® Inc. (ASX:GID), a medical device company that is developing EndoBarrier® for patients with type 2 diabetes and obesity, is pleased to announce positive EndoBarrier data from the University of Freiburg in Germany showing improvements of cardiovascular risk (CV) biomarkers and predicted 4-year risk of major CV events.
The study “Duodenal-Jejunal Bypass Liner (DJBL) Improves Cardiovascular Risk Biomarkers and Predicted 4-Year Risk of Major CV Events in Patients with Type 2 Diabetes and Metabolic Syndrome,” published by Natascha Roehlen, MD, of the University of Freiburg, demonstrates CV risk decreased significantly during EndoBarrier implantation and sustained improvements within 6 months after explantation.
Between 2012 and 2017, 71 patients diagnosed with type 2 diabetes who presented with metabolic syndrome (MS)[1] were implanted with EndoBarrier. Patient implant duration ranged from 9 to 12 months, with 45 patients completing a follow-up period ranging from 3 to 6 months after explantation. Results show a statistically significant improvement in HbA1c, BMI, fasting plasma glucose, HOMA score (insulin resistance), total cholesterol and Lp-PLA2 (cardiovascular biomarker) during the implant period.
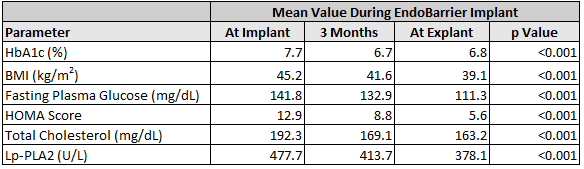
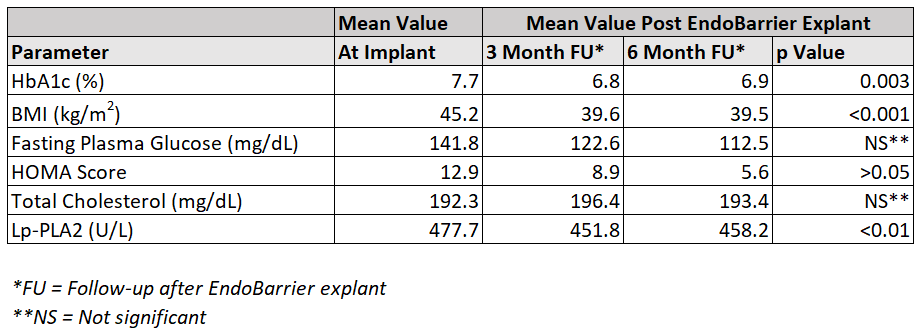
At 6 months post-explant, HbA1c, BMI, fasting plasma glucose, HOMA score and Lp-PLA2 remained below baseline. Total cholesterol changed in a significant manner during the implant period and was slightly above baseline at 6 months post-explant.
Overall CV risk was estimated by the ADVANCE Risk Engine[2] at the time of EndoBarrier implant, explant and 6 months post-explant. The data show overall CV risk decreased significantly following EndoBarrier implantation and remained stable at 6 months post-explant.
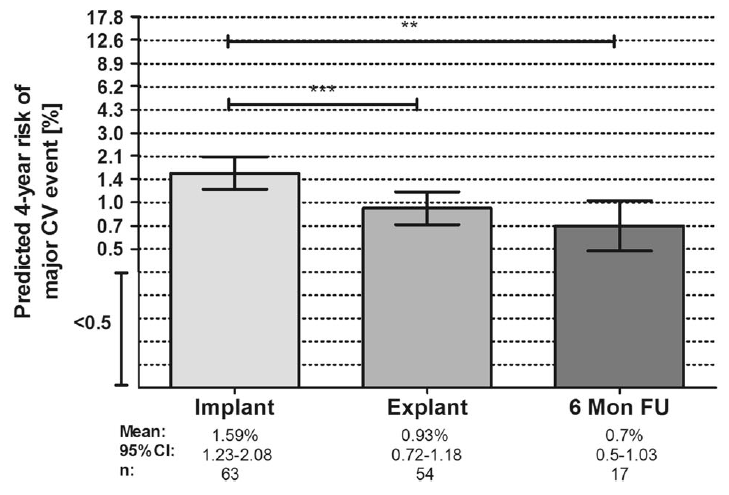
** = p Value<0.01; *** = p Value<0.001
Above data taken from the published study through Springer Science + Business Media, LLC[3]
During the EndoBarrier implant period, ADVANCE Risk Engine values for risk prediction decreased significantly, leading to an absolute risk reduction of 1.5%, with a relative risk reduction of 16.2%. Most notably, the predicted 4-year risk of major CV events remained below baseline levels within the 6 months post-explant.
“The significant improvement in HbA1c, BMI and other health-related metrics shows positive outcomes for EndoBarrier in a clinical setting,” said Stephen Linhares, vice president of clinical and regulatory affairs of GI Dynamics. “This data supports the efficacy of EndoBarrier for a multitude of health-related risks that are associated with the type 2 diabetes and obesity disease state.”
About GI Dynamics
GI Dynamics®, Inc. (ASX:GID) is the developer of EndoBarrier®, the first endoscopically-delivered medical device for the treatment of type 2 diabetes and obesity. EndoBarrier is not approved for sale and is limited by federal law to investigational use only. EndoBarrier is subject to an Investigational Device Exemption by the FDA in the United States and is entering concurrent pivotal trials in the United States and India. Founded in 2003, GI Dynamics is headquartered in Boston, Massachusetts. For more information please visit.
Forward-Looking Statements
This announcement may contain forward-looking statements. These statements are based on GI Dynamics management’s current estimates and expectations of future events as of the date of this announcement. Furthermore, the estimates are subject to several risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements.
These risks and uncertainties include, but are not limited to, risks associated with our ability to continue to operate as a going concern; our ability to obtain stockholder approval of the conversion feature of the August 2019 Note and issuance of the August 2019 Warrant, our ability to raise sufficient additional funds to continue operations and to conduct the planned pivotal trial of EndoBarrier in the United States (STEP-1); our ability to execute STEP-1 under FDA’s Investigational Device Exemption; our ability to enlist clinical trial sites and enroll patients in accordance with STEP-1; the risk that the FDA stops STEP-1 early as a result of the occurrence of certain safety events or does not approve an expansion of STEP-1; our ability to enroll patients in accordance with I-STEP; our ability to secure a CE Mark; our ability to maintain compliance with our obligations under our existing convertible note and warrant agreements executed with Crystal Amber, including our obligations to make payment on the Note that is due on 31 March 2020 and our ability to restructure the terms of the Note with Crystal Amber that is due on 31 March 2020 if we are unable to raise sufficient funds to enable us to fully repay such Note when due; obtaining and maintaining regulatory approvals required to market and sell our products; the possibility that future clinical trials will not be successful or confirm earlier results; the timing and costs of clinical trials; the timing of regulatory submissions; the timing, receipt and maintenance of regulatory approvals; the timing and amount of other expenses; the timing and extent of third-party reimbursement; intellectual-property risk; risks related to excess inventory; risks related to assumptions regarding the size of the available market; the benefits of our products; product pricing; timing of product launches; future financial results; and other factors, including those described in our filings with the U.S. Securities and Exchange Commission.
Given these uncertainties, one should not place undue reliance on these forward-looking statements. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information or future events or otherwise, unless we are required to do so by law.
###
[1] Referenced in this study, MS is a global health condition that emerges as the leading cause of cardiovascular disease (CVD) and mortality, affecting 34% of the Western population.
[2] A recently developed cardiovascular risk prediction model that enables prediction of a four-year risk of major cardiovascular events by determination of clinical parameters as pulse pressure, lipid status and HbA1c.
[3] https://link.springer.com/article/10.1007/s11695-019-04324-2

