BOSTON and SYDNEY — 22 June 2020 — GI Dynamics® Inc. (ASX:GID) (“GID” or the “Company”), a medical device company that is developing EndoBarrier® for patients with type 2 diabetes and obesity, announces that it held its Special Meeting of Stockholders on 21 June 2020 and, in accordance with ASX Listing Rule 3.13.2, is pleased to confirm that each of the resolutions put to stockholders as set forth in the Notice of Special Meeting and Proxy Statement dated 26 May 2020 (EST) (“Proxy Statement”), including a resolution to approve the proposed delisting of the Company from the Official List of the Australian Securities Exchange (“ASX”) (“Official List”), were passed.
The information required under ASX Listing Rule 3.13.2 and section 251AA of the Corporations Act is attached.
Update on Delisting from the Official List
As first announced on 11 May 2020, the Board of Directors of the Company has for some time been considering whether it is in the best interests of the Company and its stockholders to remain listed on the Official List.
For the reasons set out in the Proxy Statement and in a number of recent announcements, the Company applied to ASX for their approval to delist the Company from the Official List. In accordance with ASX’s usual practice, ASX requested, amongst other conditions, that the Company obtain the approval of stockholders by way of a special resolution to delist the Company from the Official List.
As detailed above, the Company subsequently sought, and has now obtained, the relevant stockholder approval required in order to delist from the Official List.
With the approval now obtained, and a formal delisting application already submitted to ASX, the Company will be delisted from the Official List in accordance with the timetable below.
Timetable to Delisting
The timetable for the Delisting is set out below.
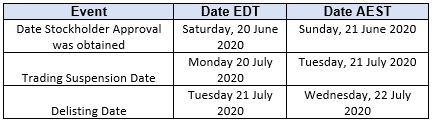
Arrangements in place to sell CDIs in the lead up to the Delisting
The Company is not in a position to operate a share buy-back or similar facility in connection with the Delisting. Stockholders that wish to sell their CDIs on ASX will need to do so before the time at which the Company’s CDIs are suspended from trading on the Official List, being the time noted in the above timetable (the “Trading Suspension Date”).
If CDI holders do not sell their CDIs prior to the Trading Suspension Date, their CDIs will need to be converted to shares of common stock in the Company. Further details on this process will be provided in the lead up to the Delisting Date.
Update on Ongoing Funding Arrangements
As has been announced by the Company on a number of occasions, the Company will need to raise additional funds to those secured under the recent Bridge Note (as announced on 19 June 2020) in order to continue to implement its business plan and to continue to carry on business. If the necessary funds are not raised, the Company may need to cease business operations and be wound up.
Further to the announcement of 19 June 2020, the Company will continue to update stockholders should there be any material developments in relation to a further fundraising.
This announcement is being made in accordance with Rule 135c of the Securities Act of 1933, as amended, and is not intended to and does not constitute an offer to sell nor a solicitation for an offer to purchase any securities of the Company.
This announcement has been authorized for release by Charles Carter, chief financial officer and corporate secretary of GI Dynamics.
About GI Dynamics
GI Dynamics®, Inc. (ASX:GID) is the developer of EndoBarrier®, the first endoscopically delivered medical device for the treatment of type 2 diabetes and the reduction of obesity. EndoBarrier is not approved for sale and is limited by federal law to investigational use only. EndoBarrier is subject to an Investigational Device Exemption by the FDA in the United States and is entering concurrent pivotal trials in the United States and India.
Founded in 2003, GI Dynamics is headquartered in Boston, Massachusetts. For more information please visit the Company website at www.gidynamics.com.
Forward Looking Statements
This announcement may contain forward-looking statements. These statements are based on management’s current estimates and expectations of future events as of the date of this announcement. Furthermore, the estimates are subject to several risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements.
These risks and uncertainties include, but are not limited to, risks associated with the Company’s ability to continue to operate as a going concern; the ability of the Company, its critical vendors, and key regulatory agencies to resume operational capabilities subsequent to the removal of COVID-19 pandemic restrictions; the Company’s ability to raise sufficient additional funds to continue operations, including the successful closing of the Potential Financing and a delisting from the ASX; the Company’s ability to execute STEP-1 under the FDA’s Investigational Device Exemption; the Company’s ability to enlist clinical trial sites and enroll patients in accordance with STEP-1; the risk that the FDA stops STEP-1 early as a result of the occurrence of certain safety events or does not approve an expansion of STEP-1; the Company’s ability to enroll patients in accordance with I-STEP; the Company’s ability to secure a CE Mark; the Company’s ability to maintain compliance with its obligations under its existing convertible note and warrant agreements executed with Crystal Amber, including its obligations to make payment on the convertible note that is now due on 29 June 2020 and its ability to further restructure the terms of such convertible note with Crystal Amber if the Company is unable to raise sufficient funds to enable it to fully repay such convertible note when due; obtaining and maintaining regulatory approvals required to market and sell the Company’s products; the possibility that future clinical trials will not be successful or confirm earlier results; the timing and costs of clinical trials; the timing of regulatory submissions; the timing, receipt and maintenance of regulatory approvals; the timing and amount of other expenses; the timing and extent of third-party reimbursement; intellectual-property risk; risks related to excess inventory; risks related to assumptions regarding the size of the available market; the benefits of the Company’s products; product pricing; timing of product launches; future financial results; and other factors, including those described in the Company’s filings with the SEC.
Given these uncertainties, one should not place undue reliance on these forward-looking statements. The Company does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information or future events or otherwise, unless it is required to do so by law.
###

